A new, less invasive approach for replacing malfunctioning heart valves is now recommended for certain patients, and younger patients can now receive natural tissue valves instead of mechanical ones, according to new treatment guidelines.
The recommendations released Wednesday by the American Heart Association and American College of Cardiology affect some of the estimated 5 million Americans with heart valve disease. The disease can be present at birth or result from infections, heart attacks or other forms of heart disease.
Major advances have taken place in the diagnosis and treatment of aortic and mitral valve disease since the last guidelines in 2014, said Robert Bonow, M.D., professor of cardiology at Northwestern University Feinberg School of Medicine in Chicago and an author of the new guidelines.
Four valves control blood flow into and through the heart. Diseased valves can lead to a backflow of blood or complete blockage of blood flow.
Historically, open-heart surgery was the only option for replacing a faulty valve. But the new guidelines endorse broadening the use of a procedure approved by the Food and Drug Administration in 2011.
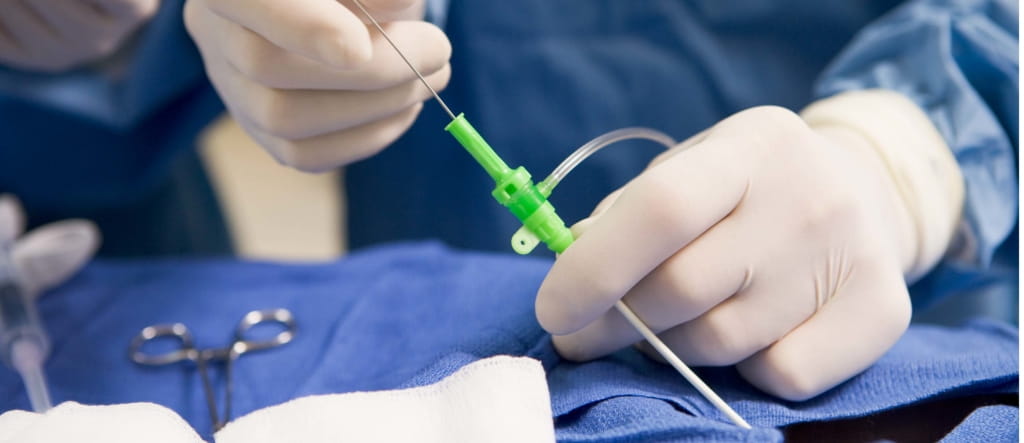
Called transcatheter aortic valve replacement, or TAVR, the procedure is recommended for people with severe narrowing of the aortic heart valve that is causing symptoms, such as shortness of breath and fatigue, and for whom open-heart surgery is too risky. But it can also be an option for some patients at lower surgical risk, said Catherine Otto, M.D., co-chair of the guidelines writing committee and a professor of medicine at the University of Washington in Seattle.
The 2014 guidelines primarily recommended TAVR to extend the lives of patients considered too sick for surgery or who faced a high risk of surgical complications. The new guidelines now provide this option to patients at lower surgical risk.
The procedure involves replacing the valve via a catheter threaded through a blood vessel.
Also new to the guidelines is the option for people as young as 50 who require surgery to replace a diseased aortic or mitral valve to receive a valve made of natural animal tissue as opposed to a mechanical valve made of artificial materials. Previous guidelines said patients had to be at least 60 to receive a tissue valve.
Mechanical heart valves typically don’t need to be replaced and were previously recommended for younger patients who needed a valve that could last decades. But mechanical valves require a lifetime of taking the blood thinner warfarin, known by the brand name Coumadin.
Although warfarin is inexpensive, it requires frequent blood tests, dietary restrictions and possibly limiting physical activity, Otto said.
The guidelines say patients and doctors should work together to decide which type of procedure and valve is best for the patient.
Avoiding warfarin is one reason many people in need of a new heart valve prefer natural tissue valves, Bonow said.
But getting a tissue valve, also called a biologic valve, at a younger age means patients will eventually need a new one, Otto said.
“If you’re 50 and get a biologic valve, you’ll need [a new] one before 70,” she said.
In the end, it comes down to the patient’s personal preference. For example, some patients may want to avoid multiple surgeries and are therefore willing to take warfarin, Otto said.
“Others would rather have multiple procedures to avoid taking anticoagulant drugs,” she said.
One challenge doctors face in helping patients make that decision is that it’s not yet clear precisely how long valves placed during a TAVR procedure last.
“Most studies have followed the newer TAVR valves for two to three years, well before the valves begin to break down,” Otto said. “Most of the time, we would not expect to see deterioration in that time span.” With surgery, however, valves last for more than 10 years in nearly all patients, she said.
Bonow has seen surgical tissue valves last as long as 20 years, although he said the valves last longer in older patients than in younger ones.
Regardless of the valve type, the guidelines recommend antibiotics before dental procedures for people who have had their heart valves replaced – as well as those who’ve had a valve repaired, a new addition to the guidelines. That’s because bacteria can enter the bloodstream during dental treatments and these patients face a higher risk of endocarditis, a bacterial infection of the lining of the heart and heart valves.
But patients shouldn’t avoid the dentist’s office, Otto said, because regular cleanings can reduce the risk of heart valve infection.
Another potential complication in people with heart valve disease is atrial fibrillation, a type of irregular heartbeat that increases the risk for blood clots and stroke. Previous guidelines recommended warfarin for AFib, but newer blood thinners may also be used, the new guidelines say.
Some patients are eager to switch from warfarin to a newer blood thinner, Otto said, but insurance providers do not always cover the switch because the newer drugs are more expensive. She hopes the new recommendations convince insurers to cover the novel drugs.
The guidelines were published in Circulation and the Journal of the American College of Cardiology.